名稱(chēng):毛細(xì)管柱
型號(hào):HH-PLOT AL2O3
規(guī)格:30m*0.53mm*15um
固定相:三氧化二鋁
*高使用溫度:200°C
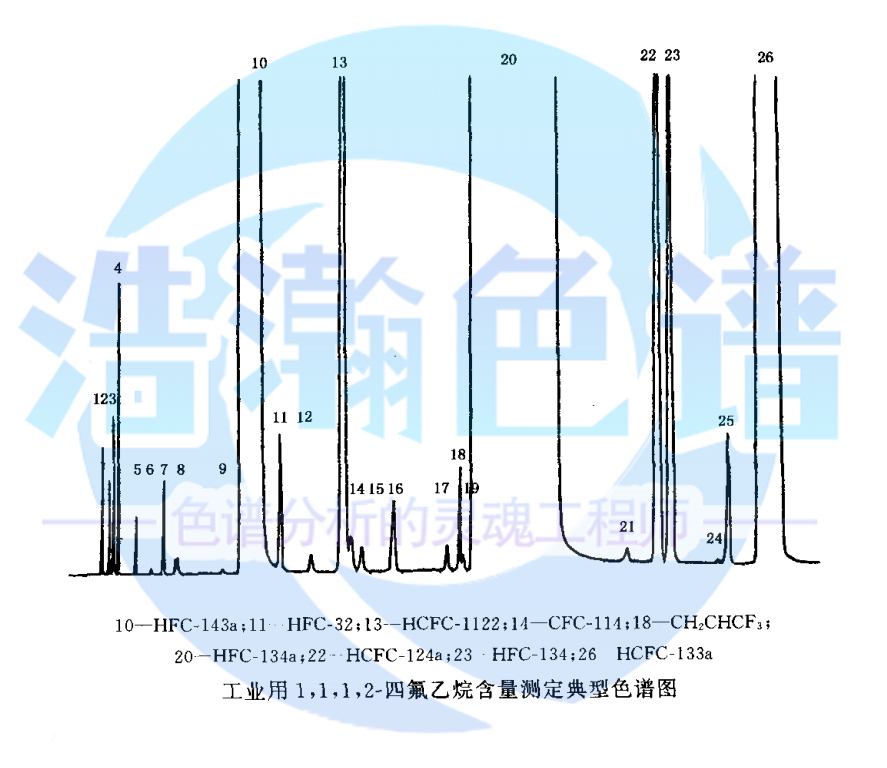
應(yīng)用:2020年版藥典中1,1,1,2-四氟乙烷的純度分析
浩瀚色譜(山東)應(yīng)用技術(shù)開(kāi)發(fā)有限公司建立了對(duì)醫(yī)用HFC-134a產(chǎn)品分析的標(biāo)準(zhǔn)方法�。 本論文包括以下三個(gè)方面: 1、醫(yī)用HFC-134a中微量CFCs雜質(zhì)定性����、定量分析。實(shí)驗(yàn)選擇HH-PLOTAl_2O_3 S毛細(xì)管柱,GC-MS定性分析醫(yī)用HFC-134a樣品中的微量雜質(zhì);以FID為檢測(cè)器,定量測(cè)定了樣品中雜質(zhì)的含量���。測(cè)試了該方法對(duì)CFCs雜質(zhì)的檢出限(0.2~0.7μL·L~(-1))���、精密度(RSD%≤6.3%)����、回收率(90.0%~120.0%)�����、線性范圍(1.0~10~5μL·L~(-1))以及線性相關(guān)系數(shù)(R~2≥0.9990)���。



Name: capillary column
Model: HH-PLOT AL2O3
Specification: 30m * 0.53mm * 15um
Stationary phase: aluminum oxide
*High service temperature: 200 ° C
Application: purity analysis of 1,1,1,2-tetrafluoroethane in Pharmacopoeia 2020
Haohan Chromatography (Shandong) Applied Technology Development Co., Ltd. has established a standard method for the analysis of medical HFC-134a products. This paper includes the following three aspects: 1. Qualitative and quantitative analysis of trace CFCs impurities in medical HFC-134a. Experiment selection HH-PLOTAl_ 2O_ 3 S capillary column, GC-MS qualitative analysis of trace impurities in medical HFC-134a samples; The content of impurities in the sample was determined quantitatively with FID as detector. The detection limit of this method for impurities in CFCs was tested (0.2~0.7 μ L · L~(- 1)), precision (RSD% ≤ 6.3%), recovery (90.0%~120.0%), linear range (1.0~10~5 μ L · L~(- 1)) and linear correlation coefficient (R~2 ≥ 0.9990).
滕州市浩瀚色譜儀器技術(shù)服務(wù)有限公司
電話:0632-5667636
傳真:0632-5667636
手機(jī):15562228838,13963221227
地址:山東滕州市平行路(商務(wù)部和技術(shù)部)
郵箱:wangxiaoying9@126.com
QQ:1404939462
聯(lián)系人:王經(jīng)理
網(wǎng)址:www.shopjjdr.com