中國藥典2020年版二氧化碳中對碳氫化合物的含量有著嚴(yán)格的控制指標(biāo)。
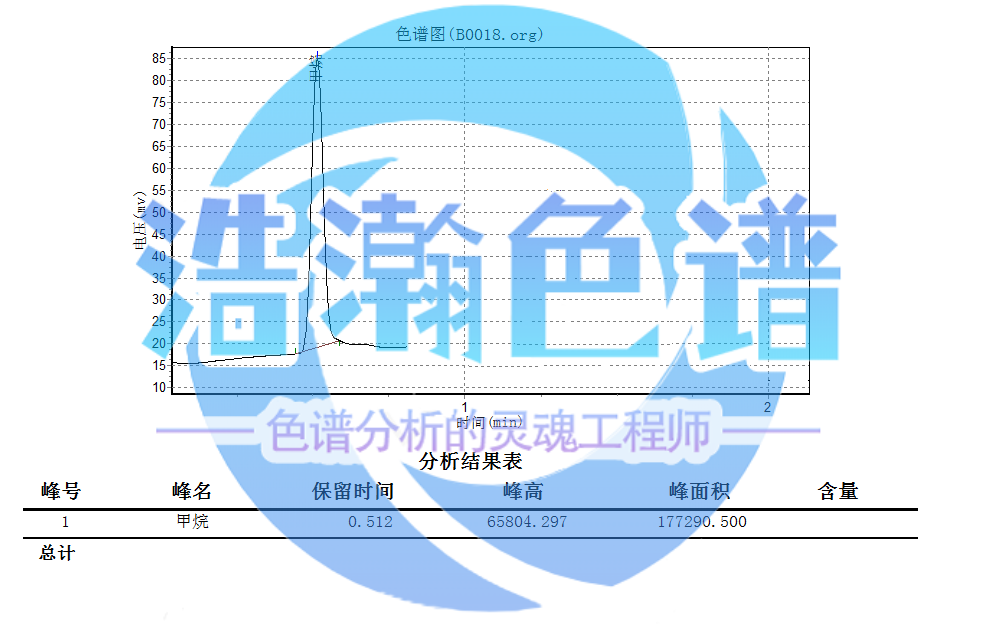
碳氫化合物照氣相色譜法(通則0521)測定供試品氣體取本品,即得對照品氣體取甲烷含量為0.0020%的氣體(以氮氣為稀釋劑)��。色譜條件用玻璃球為填料的色譜柱(4mm×0.8m,80目);柱溫為110℃;進樣口溫度為110℃;檢測器為火焰離子化檢測器,溫度為120℃測定法精密量取供試品氣體與對照品氣體,分別注入氣相色譜儀,在凈化溫度為360℃時測得的峰面積為相應(yīng)空白值;精密量取供試品氣體與對照品氣體,分別注入氣相色譜儀,測定峰面積,減去相應(yīng)空白值后的峰面積為校正峰面積限度按外標(biāo)法以校正峰面積計算,含碳氫化合物(以甲烷計)不得過0.0020%����。
浩瀚色譜(山東)應(yīng)用技術(shù)開發(fā)有限公司,與時俱進��,在王經(jīng)理的帶領(lǐng)下��,根據(jù)《中華人民共和國藥典2020第四部》中二氧化碳的要求�,成功的研制生產(chǎn)新一代HHO-III Purification device二氧化碳凈化裝置,在安捷倫Agilent7820A����,7890B,8860,8890���,島津ShimadzuGC-2010���,GC-2014等氣相色譜儀上安裝測試,結(jié)果滿意。此款產(chǎn)品在制藥單位安裝使用����,效率高,效果滿意��。
名稱:藥典二氧化碳(CO2)凈化裝置
型號:HHO-III Purification device
應(yīng)用:2020版中國藥典二氧化碳中碳氫化合物測定 氣相色譜法





China Pharmacopoeia 2020 edition carbon dioxide has strict control indicators for hydrocarbon content.
Hydrocarbons shall be determined by gas chromatography (general rule 0521), and the gas of the test sample shall be taken as this sample, that is, the reference gas shall be taken as the gas with methane content of 0.0020% (nitrogen as diluent). Chromatographic conditions: chromatographic column with glass ball as packing (4mm × 0.8m, 80 mesh); The column temperature is 110 ℃; The sample inlet temperature is 110 ℃; The detector is a flame ionization detector. The temperature is 120 ℃. The determination method accurately measures the test sample gas and the control sample gas and injects them into the gas chromatograph respectively. The peak area measured at the purification temperature of 360 ℃ is the corresponding blank value; Accurately measure the test sample gas and the control sample gas, inject them into the gas chromatograph respectively, and measure the peak area. The peak area after subtracting the corresponding blank value is the limit of the corrected peak area. The external standard method is used to calculate the corrected peak area, and the hydrocarbon content (calculated by methane) shall not exceed 0.0020%.
Haohan Chromatography (Shandong) Applied Technology Development Co., Ltd., keeping pace with the times, under the leadership of Manager Wang, has successfully developed and produced a new generation of HHO-III Purification device carbon dioxide purification device according to the requirements of carbon dioxide in the Pharmacopoeia of the People's Republic of China 2020 Part IV, and installed and tested on Agilent 7820A, 7890B, 88608890, Shimadzu GC-2010, GC-2014 and other gas chromatographs with satisfactory results. This product has been installed and used in pharmaceutical companies with high efficiency and satisfactory effect, and is highly praised by users.
Name: Pharmacopoeia carbon dioxide (CO2) purification device
Model: HHO-III Purification device
Application: Determination of hydrocarbons in carbon dioxide in Chinese Pharmacopoeia 2020 by gas chromatography
滕州市浩瀚色譜儀器技術(shù)服務(wù)有限公司
電話:0632-5667636
傳真:0632-5667636
手機:15562228838,13963221227
地址:山東滕州市平行路(商務(wù)部和技術(shù)部)
郵箱:wangxiaoying9@126.com
QQ:1404939462
聯(lián)系人:王經(jīng)理
網(wǎng)址:www.shopjjdr.com